Enabling innovation from first idea to impact
We bring the engineering expertise to design and develop your MedTech product—hardware or software—every step of the way.
Find solutions built for your success
Choose a focus area to explore how we help you grow and scale.
Integrated expertise for end-to-end MedTech development
At Factor 7, we help MedTech companies bring innovative products to market with confidence through end-to-end support across engineering, design, software, regulatory, quality, and project management. Whether you're building complex hardware, software, or connected systems, we deliver user-centered, compliant solutions that accelerate development, reduce risk, and ensure lasting success.
Designing & Engineering MedTech Innovation
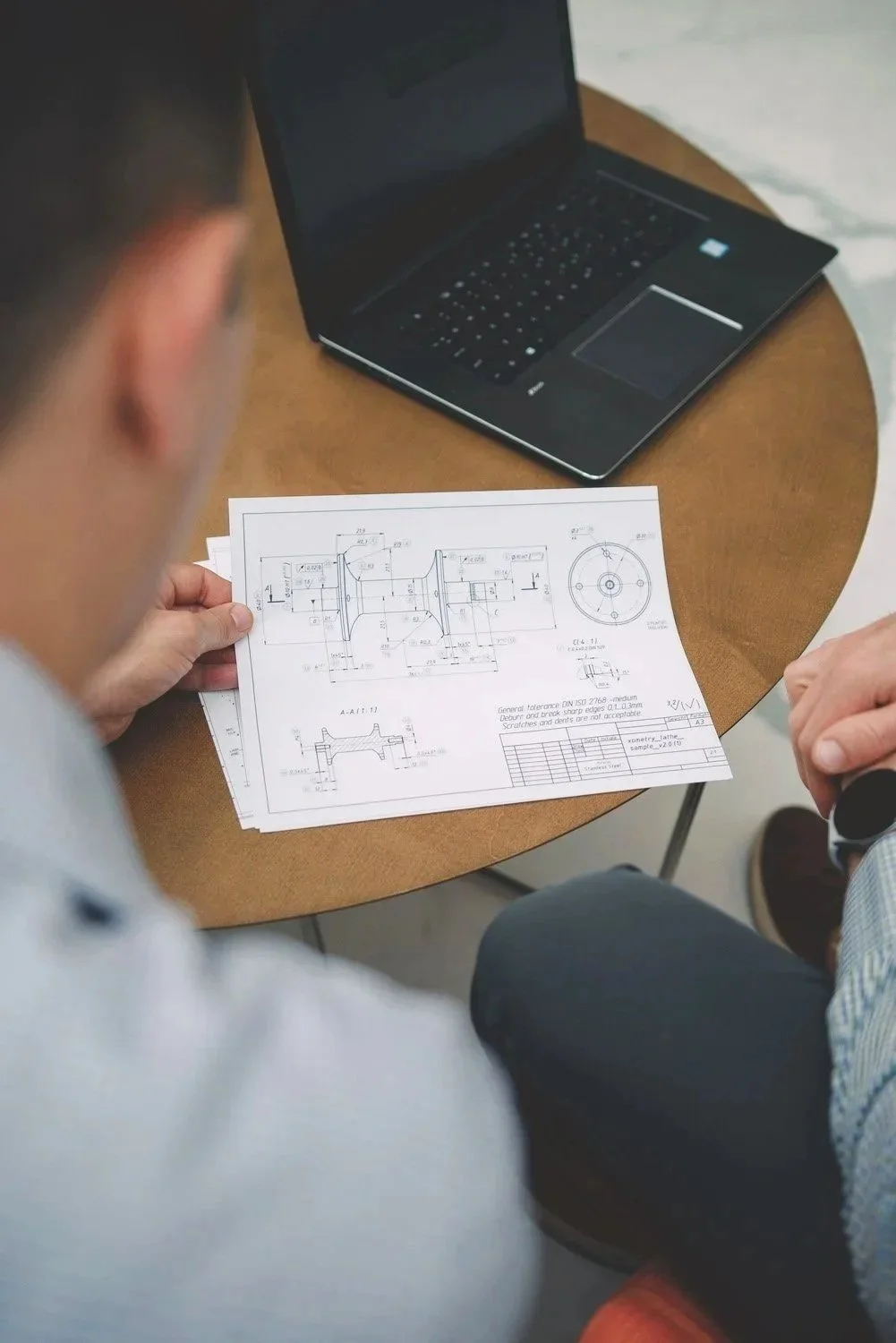
Factor 7 transforms medical device concepts into commercial-ready solutions with expert design, engineering, and full development support. From defining user needs and requirements to rapid design loop iterations and V&V testing, our development process accelerates timelines, reduces risk, and controls manufacturing costs.
Services Include:
User Need and Design Input Development: Engagement with key stakeholders at the beginning of a project to identify user needs and process into verifiable design inputs.
3D CAD Modeling and Drawings: 3D modeling using industry standard CAD programs (SolidWorks, Creo, NX, and nTopology) from initial design concepts to final manufacturing models and drawings.
Finite Element Analysis (FEA): FEA simulation of mechanical loading scenarios to optimize design performance and reduce weight in non-load bearing regions.
Design for Manufacturability and Assembly (DFMA): Designing to improve manufacturability, reduce COGS, and ensure assembled components fit and function together.
Prototyping: Sourcing and managing prototype manufacturing through our network of trusted external prototype partners for quick design iterations.
Testing: Performing and managing verification & validation testing through our network of accredited external testing partners.
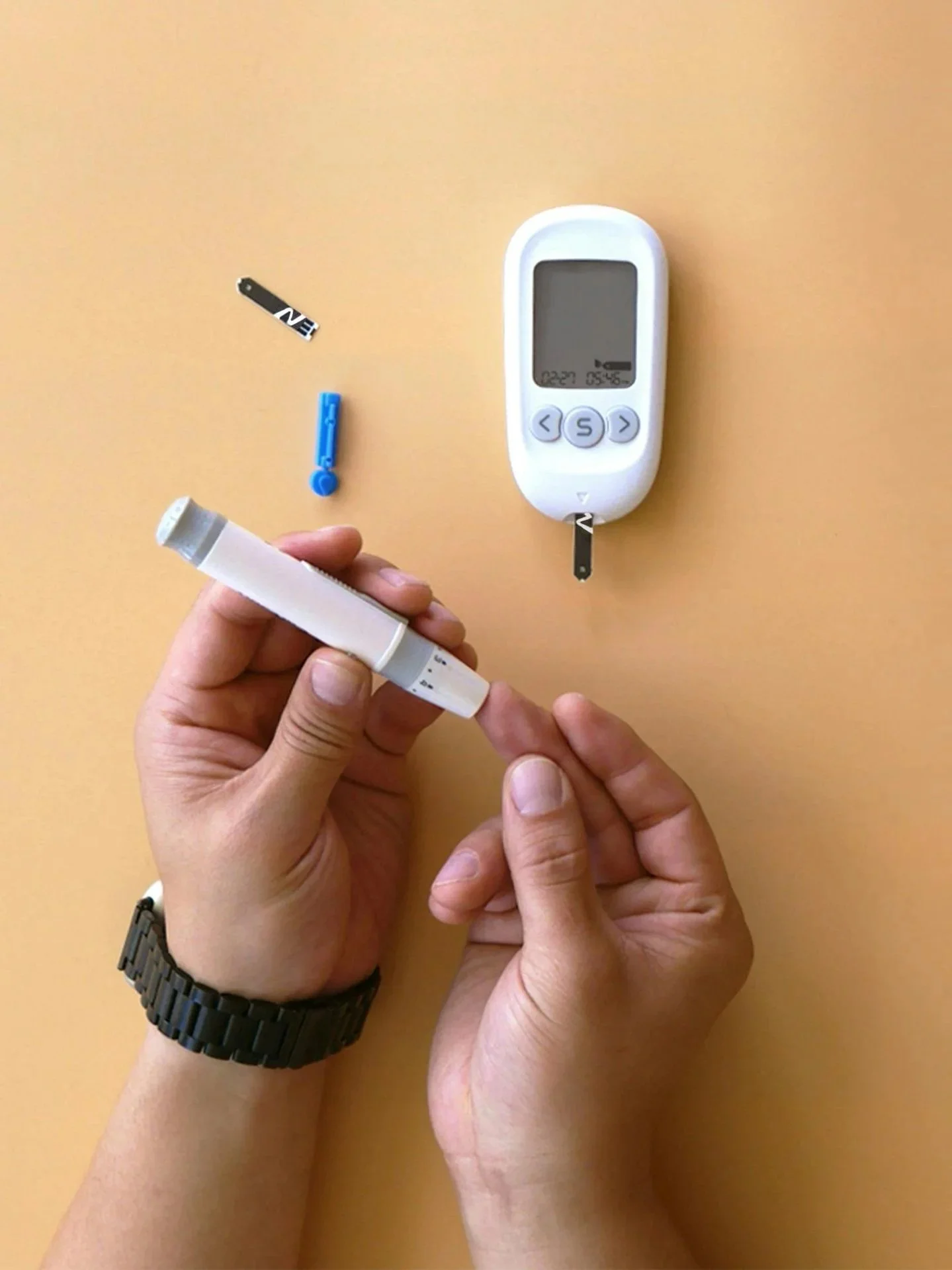
Software and Systems Design for HealthTech
Innovations leading the digital health revolution like AI, IoT, and cloud computing are making medical technologies even more complex. Factor 7 specializes in supporting the design and development of integrated software and hardware platforms across multiple healthcare applications.
Engaging Factor 7 ensures holistic systems thinking and a well-defined solution from requirements to architecture to implementation, addressing all relevant clinical, operational, security, compliance, and regulatory considerations.
Services Include:
User Needs and Software Specification Development: Analysis of key stakeholders to identify user needs and software requirements.
Software MVP Development: Rapid deployment of a minimum viable product (MVP) using an agile approach (Figma design, wire framing, workflow mapping, and Oz testing).
Software Design and Development: Development of software as a medical device (SaMD), applications, embedded firmware, SaaS, AI, or cloud connected software working alongside a device.
Artificial Intelligence (AI) Incorporation: Incorporation of AI algorithms and machine learning.
Cybersecurity Implementation: Implementation of cybersecurity to meet compliance requirements.
Systems and Architectural Design: High-level structure and organization of back-end / front-end systems to be simple, efficient, and reliable, including detailed systems design with well-defined components and interfaces.
Focusing on User Experience in HealthTech
Factor 7 understands that delivering clinical value and patient safety requires a deep understanding of user experience, human factors, and validated user-centered design. We offer a design process which addresses the many ways stakeholders may interface with the product.
Our approach allows medical and healthcare technologies—whether hardware, software, or hybrid—align with end-to-end user workflows which optimize the user experience to drive clinical efficiency and adoption.
Services Include:
User Analysis: Identification and analysis of all stakeholders involved in the use of a product.
User Workflow Study: Review of end-to-end user workflow and user experience scenarios.
Human Factors Design: Creation of user-centered system requirements and interface optimization between the product and the user, whether it is software or a mechanical device.
Usability Testing: Performing and managing validation testing ensuring the overall user experience needs are met.
Quality Management for MedTech
Factor 7 helps MedTech companies build and maintain quality systems that ensure compliance, reduce risk, and enhance product performance.
Services Include:
Quality Management System (QMS) Development & Compliance: Establishment or optimization of QMS frameworks to meet regulatory and operational requirements: ISO 13485, FDA 21 CFR Part 820, EU MDR/IVDR, GMP, & cGMP.
Internal & External Audits: Preparing organizations for FDA, ISO, and supplier audits and closing gaps with corrective action plans.
Risk Management & Process Validation: Failure Modes and Effects Analysis (FMEA & PFMEA), process validation (IQ, OQ, PQ), and Corrective and Preventive Actions (CAPA).
Supplier Quality Management: Supplier qualification & audits, incoming Inspection & control plans, and Supplier Corrective Action Requests (SCAR).
Continuous Improvement & Lean Six Sigma: Lean manufacturing, Six Sigma (DMAIC Approach), and Statistical Process Control (SPC) for real-time quality monitoring.
Complaint Handling & Post-Market Surveillance: FDA MDR (Medical Device Reporting) compliance, Root Cause Analysis (RCA), and complaint trending and risk-based response systems in post-market Surveillance plans.
Regulatory Affairs for MedTech
Factor 7 guides medical device companies through every stage of the regulatory process, from early planning to post-market surveillance. With strategic expertise and technical insight, we streamline submissions, reduce risk, and ensure global compliance for successful market entry.
Services Include:
Regulatory Assessment: Assessment of appropriate regulations, product code, classification, potential predicates, likely testing, and realistic timeline to submission.
Regulatory Pre-Submissions: Planning and navigation of pre-submission questions and responses.
Regulatory Strategy: Final strategy for regulatory submission including product code, classification, predicates, and required testing.
Regulatory Submission: US FDA: 510(k), De Novo, and PMA submissions. In Europe: CE Mark submission. Submissions to other regulatory bodies outside of US and EU are also supported.
Post-Market Surveillance: Planning and navigation of post-market surveillance to meet regulatory requirements or collect data to support further claims and submissions.
Project Management for MedTech
At Factor 7, we provide expert project management to guide medical device development from concept to commercialization. We align cross-functional teams, manage risks, and keep projects on schedule, within budget, and in compliance.
Factor 7 delivers disciplined and efficient project execution, enabling MedTech companies to advance innovation while staying focused on their strategic objectives.
Services Include:
Project Planning and Execution: Development of comprehensive project plans, timelines, budgets, and resource allocation strategies tailored to product and business goals.
Design Control & Stage Gate Management: Integration of design control processes with formal stage gate reviews to ensure compliance and development rigor at every phase.
Cross-Functional Team Leadership: Day-to-day coordination and leadership of internal and external stakeholders across engineering, regulatory, quality, operations, marketing, and management.
Risk Management & Issue Resolution: Proactive identification, assessment, and mitigation of technical, regulatory, and operational risks throughout the development lifecycle.
Vendor & Partner Management: Oversight of third-party development partners, contract manufacturers, test labs, and service providers to maintain alignment, timelines, and quality.
Our Services
At Factor 7, we empower medical technology companies with the strategy, expertise, and execution needed to succeed—from startup to scale.
Business & Capital
Unlock your MedTech startup’s full potential with tailored strategies and expert guidance at every stage.
Product Development
Delivering user-focused, compliant MedTech solutions that accelerate development and ensure lasting success.
Sales & Marketing
Connecting your MedTech innovation to the right stakeholders to ensure lasting adoption, reimbursement, and growth.